Immunotherapy Looks to Harness the Body’s Defense and Cure Cancer from the Inside Out
As little as two decades ago early adopters of immunotherapy, looking to harness the body’s immune system to fight disease, toiled in the shadow of the holy trinity of cancer therapy — surgery, chemotherapy, and radiation therapy. Now, the immunological approach to cancer treatment has undergone a research renaissance. Times certainly have changed.
Following news of remarkable recoveries, this once-overlooked avenue to a cure for cancer has captured the public’s imagination. Oncologists have embraced immunotherapy as an important advancement, and the focus now is on refining those therapies to increase the cure rate and ameliorate potential adverse side effects.

One of the most exciting developments on the immunological front is T-cell therapies for lymphoma. T-cells are a type of lymphocyte produced or processed by the thymus gland (the T comes from thymus) that actively participate in the immune response. Typically, the most widely reported among these new treatments is CD19 CAR T-cell therapy. Doctors attach chimeric antigen receptors (CAR) to T-cells and reintroduce them in the patient to seek out cancer cells and produce chemical compounds that destroy them.
A research team led by Catherine Bollard, PhD, associate center director for translational research and innovation at the George Washington University (GW) Cancer Center, has developed a different therapeutic approach called TAA-T, which is a multi-antigen specific T-cell product that may prove to be superior in many ways to the recently FDA-approved CD19 CAR T-cell therapy. “
If you’ve got a tumor cell, there are several ways to kill it,” says Bollard, who also serves as professor of pediatrics at the GW School of Medicine and Health Sciences (SMHS) and director of the Center for Cancer and Immunology Research at Children’s Research Institute, part of Children’s National Health System (Children’s National). The CD19 CAR T-cell technique, she explains, involves genetically engineering a T-cell — adding an artificial receptor — so that it recognizes and kills the tumor. TAA-T requires no genetic engineering. Tumor-killing T-cells, present naturally in everyone, are harvested from the patient and fed with cytokines or other chemicals in the lab where technicians grow their numbers to millions. They are then re-infused into the patient. Bollard says the entire process used to take three to six months, which dimmed its luster. Now it can be completed in less than two weeks, “so it’s a much more nimble process,” she notes. Genetic modification, on the other hand, is expensive and heavily regulated and thus a cumbersome process.
TAA-T has none of these disadvantages. Moreover, CAR T is limited to recognizing proteins on the surface of the tumor cells, and that is very restricting, Bollard explains. “There are only a limited number of proteins on the surface of tumors (or ‘extracellular antigens’) you can target with CAR T,” she says, “whereas our TAA-T can target what is known as intracellular antigens — proteins within the tumor cells — and there are many more of these cancer-specific proteins that can be targeted. In addition, because CAR T targets only one antigen, the patient can develop a resistance to the therapy.” The minimal toxicity of TAA-T is perhaps its most significant advantage. Patients undergoing CD19 CAR T-cell therapy sometimes experience cytokine release syndrome, or CRS, which is caused by a large, rapid release of cytokines and cellular by-products into the bloodstream when the cancer cells are destroyed. Symptoms can vary from the relatively mild — fever, nausea, headache, rash, rapid heartbeat, low blood pressure, and trouble breathing — to the more severe. According to Bollard, CD19 CAR T-cell therapy is associated with “appreciable toxicities including neurotoxicity and, in some cases, death.”

In Phase I clinical trials with Bollard’s TAA-T therapy, researchers are now seeing 75 percent efficacy, especially in acute myeloid leukemia, which the trials with CAR T have thus far been unable to achieve. “We’re just beginning to increase the number of patients [in the trials], both adult and pediatric,” says Bollard. “We were initially collaborating with [Johns] Hopkins because the program at GW wasn’t up and running, but with Kieron’s arrival at GW we are now ready to move this therapy forward at GW for the treatment of other forms of lymphoma and even multiple myeloma.” Kieron is Kieron Dunleavy, MD, professor of medicine at SMHS and director of the lymphoma program. “We are excited for Kieron to lead the novel T-cell therapy studies at GW,” Bollard says. “We obviously see GW as a huge player in this because of Eduardo [Sotomayor, MD, director of the GW Cancer Center and professor of medicine at SMHS], Kieron, and Mitch [Smith, MD, PhD, associate center director for clinical investigations at GW Cancer Center], who are all internationally renowned lymphoma physicians.” Dunleavy came to GW in May 2017 after nearly 15 years at the National Institutes of Health working on lymphoma therapies and trials. “My goal is to improve the cure rate for the most common form of lymphoma, called diffuse large B-cell lymphoma. There are lots of exciting therapies in development, and they are really changing the face of lymphoma,” he says.
Because all cancer tumors have proteins on their surface, Bollard’s lab “trains” the T-cells by loading “trainer cells” called antigen-presenting cells with pieces of the proteins (peptides) that are expressed by the tumor cell. Then the researchers mix these peptide-loaded training cells with T-cells from the patient or a healthy donor. The T-cells are stimulated by the tumor peptides to reproduce in the laboratory, growing to large enough numbers that they can then be infused back into the patient. “These [enhanced] T-cells,” explains Bollard, “will then kill the tumor target, get stimulated, and continue to replicate in the patient. “In some ways it’s like a cancer vaccine,” she explains, “but you’re doing the job of the vaccines in the lab.”
Adult trials began in the fall of 2016, and pediatric patients joined the study in April 2017. “We started with patients who’d had a bone marrow transplant,” says Bollard. “What we want to do with Kieron is to incorporate patients who are ineligible for transplants or who weren’t going to have a transplant, and combine it with other immunotherapies — for instance, adding checkpoint inhibitors.”
Checkpoint inhibitors are molecules that block immune checkpoints, which are receptors or ligands that modulate the immune response. In many cancers, tumor cells express these checkpoints to “put the brakes on” the immune system. Blocking the immune checkpoint thus releases the brakes.
“Tumors are clever,” Bollard says with a wry grin, “and they try to resist attack from the immune system. We’ve always treated cancer with different drugs. My feeling now is we should treat cancer with different immune therapies. And as long as you don’t increase toxicity to crazy levels, it’s really enhancing the immune therapy to increase efficacy.”
Bollard opens her computer and pulls up a graphic that displays the huge number of immunotherapy projects going on around the world. It’s nearly as vast and dense as a chart of stars. For years, she explains, oncologists have thought of cancer as a disease that needed to be treated by drugs. “Those of us who were proponents of the immune system being our best defense against cancer were looked at with a pretty dim view,” she says. “Whether or not these cells are the be-all and end-all, what this means is that the community is now understanding that the immune system is really our first line of defense against cancer,” says Bollard. “We should be harnessing it early in the disease process and not waiting until we’ve killed off the immune system with multiple lines of chemotherapy and/or radiation, and then trying to get the immune system to help us.”
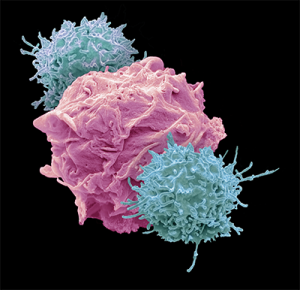
Arming the Immune System
Humans’ immune systems feature a variety of white blood cells — including macrophages, dendritic cells, and several types of lymphocytes — each with a different method for protecting the body from foreign invasion or disease. These types of white blood cells circulate to every part of the body, providing protection from cancer and other diseases. Macrophages are white blood cells that swallow and digest foreign particles and signal other immune system cells to go into action. Dendritic cells present the foreign cells to the immune system. Lymphocytes include B-cells, T-cells, and natural killer (NK) cells:
- B-cells make antibodies that tag foreign or abnormal cells so other parts of the immune system can destroy them.
- T-cells directly attack cancer cells and signal other immune system cells to defend the body.
- NK cells find, bind to, and kill foreign invaders or damaged cells in the body.
Chimeric Antigen Receptor (CAR) T-cell Therapy
The concept of engineering chimeric antigen receptors dates back about 20 years, and it has been touted as a treatment for a range of cancers. Two CAR T-cell therapies were approved by the FDA in 2017, and since then the number of immunotherapy trials has ballooned. Cytokine Release Syndrome, or CRS, occurs after CAR T-cell treatment. Symptoms such as fever, nausea, headache, or trouble breathing can range from mild to severe or even life-threatening. According to the National Cancer Institute, the more effective the CAR T-cell treatment is, the more likely a patient is to experience CRS because the therapy has destroyed so many cancer cells the body is littered with cellular debris. The rapid release of cytokines into the blood from the immunotherapy can send the immune system into a tailspin.



