A rare disease is like a unique jewel; you can examine it from different angles, and its combination of facets create an overall stunning whole. Myasthenia Gravis (MG), an autoimmune neuromuscular disease affecting roughly 160,000 Americans, is that gem in the hands of Henry Kaminski, MD, Meta A. Neumann Professor of Neurology at the George Washington University (GW) School of Medicine and Health Sciences (SMHS).
Kaminski is the MG expert at the helm of the Myasthenia Gravis Rare Disease Network, or MGNet, a research enterprise funded by a $7.8 million grant from the National Institutes of Health, as well as support from donors such as Barry Green. He’s collected global resources to examine MG from multiple angles — patient assessment technology, root causes and potential therapies — and it’s making a difference.
A Challenging Disease
MGNet, established in 2019, is one of just 22 rare disease networks working to uncover critical information for patient care and potential therapies.
“By definition, each disease is rare,” Kaminski explains. “But if you start accumulating all these [diseases], there are about 15 million people in the U.S. who have a rare disease. So, there’s significant advocacy toward understanding and improving the care of patients with rare diseases.”
What makes MG particularly tricky is that it varies in how it affects individuals. It’s hard to recognize, which means many patients go undiagnosed for several years. Researchers are also learning that MG is not one disease; rather, it has different underlying causes and mechanisms of disease that are based on age and gender as well as some racial and ethnic characteristics. There’s little known about MG subtypes, and currently, there are no known biomarkers to determine the efficacy of therapeutics.
With the grant in hand, Kaminski and colleagues around the world sought to home in on one of the components: natural history. The plan was to identify patients whom they could follow, conducting formal exams and drawing blood every six months over the course of two years. With the resulting data from the natural histories and samples, the team looked to identify markers of disease development and predict treatment outcomes.
They had everything lined up by early 2020 — and then COVID-19 hit.
“Immediately telemedicine was started across the country, and in-person research visits were stopped,” Kaminski recalls.
The research team needed to adapt, along with the rest of the world. Enter telemedicine and artificial intelligence (AI).
Medicine of the Future
The research team — as is fitting with scientists — first devised a study to evaluate the accuracy and reproducibility of telemedicine visits for research purposes.
“We began a study using recorded video visits and ultimately had 52 patients recorded at two [separate] visits, [allowing] comparisons over time,” Kaminski explains. “We had independent evaluators assess the recordings to compare ratings of weakness.”
As part of patient evaluation, researchers assessed key symptoms of MG: eyelid droop, double vision, slurred speech, and diminished arm and leg strength. They also evaluated patients’ facial muscle strength with tongue-to-cheek and cheek puff exercises.
It was around this time that Kaminski started working with Marc Garbey, PhD, a research professor of surgery at GW SMHS. Garbey is also president and founder of Care Constitution, a telehealth platform. Care Constitution offers a wide range of applications for objective, reproducible, and quantitative health care assessments for acute spinal cord injury, amyotrophic lateral sclerosis, and multiple sclerosis.
Together with Kaminski, Garbey and his team developed a hybrid application that combines deep learning with computer vision for MG symptom evaluation. The AI can measure the degree of eyelid droop, for instance, with high accuracy. In fact, Kaminski says, the tool is far more precise than the standard mild, moderate, or severe physician assessments, which can be subjective and vary from clinician to clinician.

This digital health technology (DHT) works in real time, on both a fixed image and frame-by-frame, and the data can include sleep and physical activity tracking from smartwatches or smartphones. It’s still in its early stages, but Garbey and Kaminski say it has immense potential. The DHT tool eases patient visits and, as Garbey notes, is meant to give health care professionals a reliable tool — not replace them.
“The bottom line is that the system is automatic and does not require additional work for the doctor,” he explains. “It uses standard video code. It gives another verification for the doctor.”
The implications of the tool are ground-breaking for equity and research. Garbey notes that there’s a shortage of neurologists, which can add a barrier to care. An AI tool, readily available, can assess any patient, anywhere in the world. Both Garbey and Kaminski also point out that the tool’s language is adjustable, and the avatar that interacts with patients is customizable. It can be young or old, male or female, with any race or ethnicity, all of which can potentially make patients more comfortable during an examination.
“You provide access and equity because the service, once it’s delivered, should be very cheap, and there is no reason why anybody cannot access it,” Garbey says. “Once you get this kind of management of the disease and accelerate the diagnostic [process], then the doctor can do their job.”
The tool could help detect MG in patients earlier, which could lead to quicker, more effective treatment. Plus, because it strips subjectivity from measurements, there’s consistency among assessments, a critical factor for clinical trials and FDA approval for novel treatments.
And it’s the treatment stage where the research of Linda Kusner, PhD, research professor of pharmacology and physiology at GW SMHS, comes in.
The Way to a Cure
Kusner, in collaboration with investigators from Germany and the United States, including Roswell Park Comprehensive Cancer Center in New York, is focusing on a specific protein — survivin — as a therapeutic target to change the course of the disease.
“There are similarities, somewhat, with cancers and autoimmunity in the sense that there are cells in the body that are proliferating,” Kusner says. “They’re not supposed to be there.”
With MG, B-cells produce antibodies that target the acetylcholine receptor at the neuromuscular junction, leading to muscle weakness. Many cases of early-onset MG stem from abnormalities in the thymus, the home of these antibodies.
Kusner and her team looked at lymphocytes and other B-cells — part of the immune response — in MG patients and found a consistent increase in survivin expression. To further test the link between MG and cancer, Kusner studied SurVaxM, a cancer vaccine as well as an antibody-based drug from biotechnology company MimiVax that targets survivin-expressing cells. She determined that the vaccine reduced B-cell expression and antibody levels.
“There’s the potential to develop a novel treatment,” Kusner says.
The goal, she adds, is to develop an anti-survivin-specific antibody as a therapy for MG.
“We’re still working with MimiVax to try to humanize the antibody and look deeper into whether this could be a potential treatment for patients to be using,” Kusner says. “Hopefully, we can start clinical trials, and five years from now, we’ll see whether the therapy shows a benefit and lives up to what I hope it will in terms of a cure.”
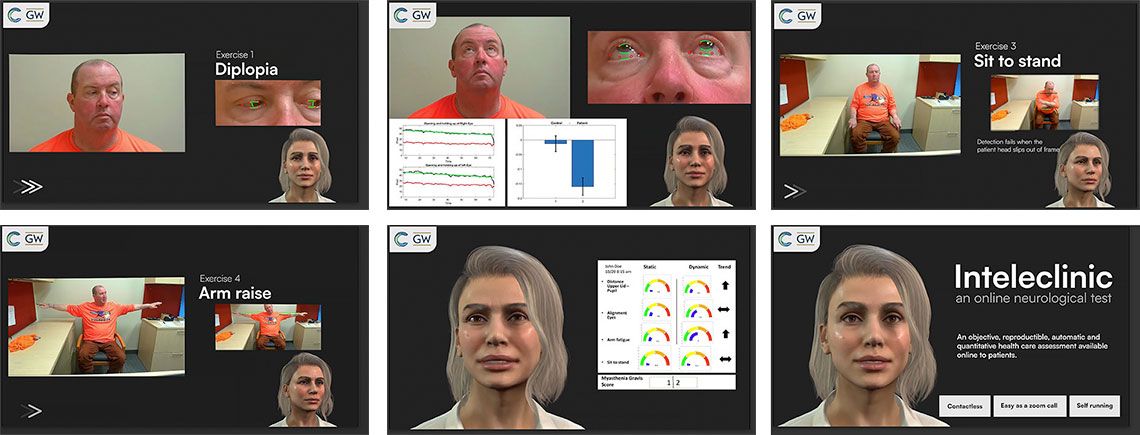
Digital Health Technology: Adding AI to the Exam Room
The online app leads patients through a series of simple exercises to assess ptosis (eyelid droop), diplopia (double vision), dysarthria (slurred speech) and arm and leg strength, and examines facial muscle strength with tongue-to-cheek and cheek puff exercises. The AI algorithm then measures the degree of muscle weakness — a hallmark of MG.
Continued Developments
While Kaminski, Garbey, and Kusner’s research is progressing, MGNet is racking up considerable additional projects — and accomplishments.
One component of the grant supports junior investigators in developing their careers. Currently, MGNet has provided funds to centers across the United States, as well as Germany, Italy, and Canada, and it has helped to fund pilot studies.
“Our scholars have been extremely successful,” Kaminski says, citing investigators narrowing in on MG research — one is studying telemedicine and remote monitoring, for instance — at Massachusetts General Hospital. Others at Duke University and Yale University are studying fundamental mechanisms of how antibodies and immune cells differ among patients.
Yale is the site of another of MGNet’s key projects. The team there is exploring the role of antibodies in disease and how they injure the neuromuscular junction in MG. There are three mechanisms in which the most common antibodies work, but as is the norm with MG, not all patients have the same mechanisms at work.
Meanwhile, the MGNet natural history study has now enrolled more than 200 patients and is growing in numbers.
“That is the largest prospective, rigorous study in the history of this rare disease,” Kaminski says.
Standardized exams and collection of immune cells, serum, and plasma will form a basis to identify markers of the disease, he adds.
All of this progress highlights one main point: For a disease that has multiple causes, multiple patient profiles, and multiple treatment responses, personalized medicine is key.
“Each patient is different,” Kaminski says. “Our goal is to do deep dives to understand each patient’s disease profile.”
Now, he says, they are — and MGNet is leading the way.




